The University of Hyogo was established in 2004 through a merger between the Kobe University of Commerce, the Himeji Institute of Technology and the College of Nursing, Art and Science Hyogo. The top-class public university comprises 6 faculties, 14 graduate schools, 4 attached laboratories, an attached high school and junior high school, and will this spring, establish the new “Graduate School of Disaster Resilience and Governance”.
Main areas of interest at the university’s Graduate School of Engineering, include things such as the benchmarking of electrolytes in ionic liquids and the study of polymerization reactions. Kyoto Electronics Manufacturing (KEM) has the great pleasure of supporting their work, through introducing to them our EMS-1000S Viscometer and our DA-100 Density/Specific Gravity Meter. To further understand the important work done at the school, we took the opportunity to conduct the following interview with Professor Takeshi Kakibe.
COLUMNIn the field

Customer Interview 1: Professor Takeshi Kakibe Ph.D., University of Hyogo, Graduate School of Engineering
School Motto:
“Manabitsuzukeru kimi ni, chikara wo!”
(“Supporting our scholars of the future”)
Official website:
https://www.u-hyogo.ac.jp/english/index.html

University of Hyogo, Graduate School of Engineering (Himeji City, Hyogo Prefecture)
About Professor Kakibe’s research into Ionic Liquids
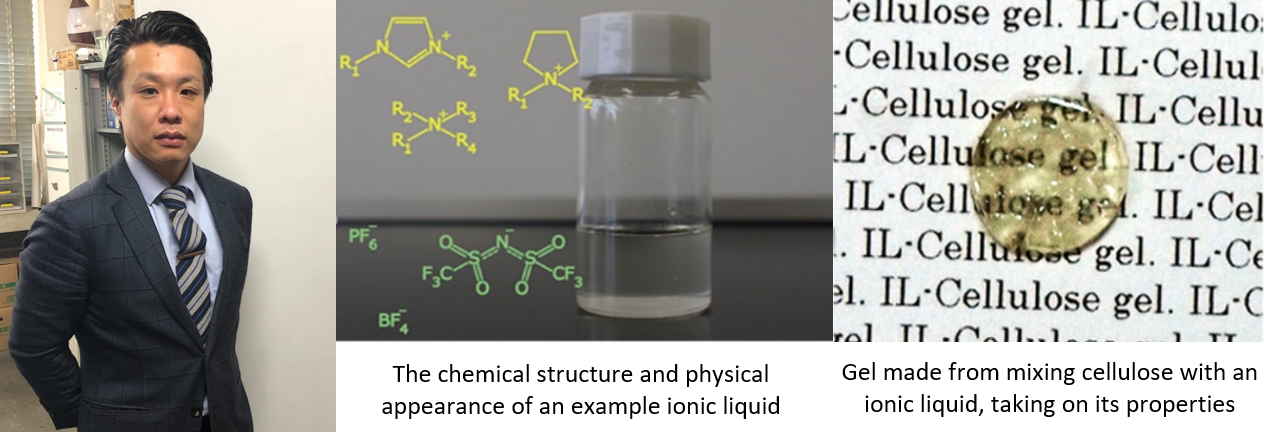
Prof. Kakibe: As significant research into ionic liquids began a mere 20 years ago, it remains a relatively new area of study. Seeing as some salts comprise of organic compounds, it is possible to use these organic salts in chemical structure design. One of the most abundant substances on Earth, cellulose, is known for its insolubility in typical solvents, though it has been found to break down readily in ionic liquids containing certain organic salts, of which it shows high chemical compatibility to.
Utilizing this knowledge, cellulose can be dissolved using low amounts of energy and modified to have very useful chemical properties, presenting new potential for this readily available organic compound. This gel (pictured) which was made with cellulose and an ionic liquid, takes on and preserves the properties of the ionic liquid and can serve as a flexible membrane. This type of material has great potential due to its functionality and safety of use, so we are enthusiastically continuing our research with view to the production of novel cellulose derivatives.
Asking Professor Kakibe about KEM’s EMS Viscometer

Uchida (KEM): Could you tell us what led you to using KEM’s Viscometer?
Prof. Kakibe: Viscometers we used in the past, had a tendency to introduce water into our samples during measurement, compromising the precision of our readings. We were also concerned about the larger amount of sample required for measurements as well as the possibility of contamination getting into samples, so upon voicing our various concerns to a KEM distributor, we were introduced to the EMS Viscometer and they soon organized a product demo for us.
Uchida: How were you feeling after your hands on demo with the EMS?
Prof. Kakibe: Very good! In the past, we were using DMA (Dynamic Mechanical Analysis), and reassuringly, the measurement values we obtained using the EMS were almost identical. As many of the samples we work with are scarce, we found KEM's EMS to be attractive as it allows us to scale down sample amounts and to recycle samples for separate analyses. On top of that, samples can be measured inside a sealed test tube, and if worked with in a glove box, we can ensure our samples are hermetically sealed.
Uchida: What has the adoption of our EMS Viscometer meant for your lab?
Prof. Kakibe: It allows not only our experienced viscometer users, but our first-timer students alike to attain reliable viscosity data.
Uchida: How has it contributed to your research?
Prof. Kakibe: Well as a matter of fact, in January this year, we published the paper, “Branched Alkyl Functionalization of Imidazolium-based Ionic Liquids for Lithium Secondary Batteries”, with research aided by the EMS Viscometer.
<Link to the paper> https://doi.org/10.5796/electrochemistry.22-00001
Uchida: Oh, what fortunate timing for this interview then (haha)! I’m most glad to hear it, congratulations.
Uchida: Next, could you tell us how our customer support has been?
Prof. Kakibe: Response times have been great, and we were able to start using the EMS quickly thanks to the quick and smooth set up process. Our recent graduates were most pleased, as the viscometer was of great use to their final studies.
Uchida: Thank you kindly for your time and telling us about your important research! We look forward to helping you in the future.